Tofacitinib citrate magani ne na magani (sunan kasuwanci Xeljanz) wanda Pfizer ya samo asali don aji na masu hana Janus kinase (JAK). Yana iya zaɓin hana JAK kinase, toshe hanyoyin JAK/STAT, kuma ta haka ya hana siginar siginar tantanin halitta da maganan jinsin da ke da alaƙa da kunnawa, waɗanda ake amfani da su don magance cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan cututtukan da da kuma sauran cututtukan da suka shafi rigakafi.
Magungunan sun hada da siffofin sashi uku: Allunan, dorewa-saki allunan da mafita na baka. FDA ta fara amincewa da allunan sa a cikin 2012, kuma FDA ta amince da sigar ci gaba-saki a watan Fabrairun 2016. Shi ne na farko don kula da gidajen abinci na rheumatoid. Yan shine mai hana JAK da ake sha da baki sau ɗaya a rana. A watan Disamba na 2019, an sake amincewa da sabuwar alamar magungunan da aka ci gaba don matsakaita zuwa mai tsanani ulcerative colitis (UC). Bugu da ƙari, an kammala gwajin gwaji na asibiti na yanzu na 3 na plaque psoriasis, kuma wani lokaci na shida na 3 na gwaji na asibiti yana ci gaba, wanda ya hada da cututtukan cututtuka na psoriatic mai aiki, ƙananan yara na idiopathic arthritis, da dai sauransu. Irin alamu. Amfanin allunan da aka ɗorewa waɗanda ke daɗe suna aiki kuma suna buƙatar shan sau ɗaya kawai a rana suna da amfani ga gudanarwa da sarrafa cututtukan marasa lafiya.
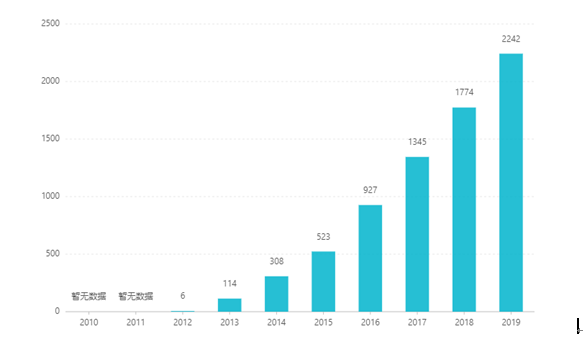
Tun lokacin da aka jera shi, tallace-tallacen sa ya karu kowace shekara, wanda ya kai dalar Amurka biliyan 2.242 a shekarar 2019. A kasar Sin, an amince da nau'in nau'in nau'in kwamfutar hannu don tallatawa a cikin Maris 2017, kuma ya shiga cikin kundin inshorar likitanci na B ta hanyar shawarwari a cikin 2019. Nasara ta ƙarshe. Farashin shine 26.79 RMB. Koyaya, saboda babban shingen fasaha na shirye-shiryen sakewa mai dorewa, har yanzu ba a sayar da wannan nau'in adadin a China ba.
JAK kinase yana taka muhimmiyar rawa a cikin kumburi, kuma an nuna masu hana shi don magance wasu cututtuka masu kumburi da autoimmune. Har zuwa yanzu, an amince da masu hana 7 JAK a duniya, ciki har da Leo Pharma's Delgocitinib, Celgene's Fedratinib, AbbVie's upatinib, Astellas's Pefitinib, Eli Lilly's Baritinib da Novartis's Rocotinib. Duk da haka, tofacitinib, baritinib da rocotinib ne kawai aka amince da su a kasar Sin daga cikin magungunan da aka ambata a sama. Muna sa ran za a amince da "Tofatib Citrate Sustained Release Allunan" na Qilu da wuri-wuri kuma yana amfana da ƙarin marasa lafiya.
A kasar Sin, NMPA ta amince da ainihin binciken tofacitib citrate a cikin Maris 2017 don kula da marasa lafiya na RA masu girma tare da rashin isasshen inganci ko rashin haƙuri ga methotrexate, a ƙarƙashin sunan kasuwanci Shangjie. Bisa kididdigar da aka samu daga Meinenet, sayar da allunan tofacitib citrate a cibiyoyin kiwon lafiyar jama'a na kasar Sin a shekarar 2018 ya kai yuan miliyan 8.34, wanda ya yi kasa da yadda ake sayar da shi a duniya. Babban ɓangare na dalili shine farashin. An bayar da rahoton cewa, farashin sayar da kayayyaki na farko na Shangjie ya kai yuan 2085 (kwallolin 5mg*28), kuma farashinsa a kowane wata ya kai yuan 4170, wanda ba karamin nauyi ba ne ga iyalai na gari.
Duk da haka, yana da kyau a yi bikin cewa an haɗa tofacitib a cikin 2019 "National Basic Medical Insurance, Work Insury Insurance and Maternity Insurance Drug List" da Hukumar Inshorar Lafiya ta Kasa ta yi bayan tattaunawa a watan Nuwamba 2019. An ruwaito cewa za a rage kudin kowane wata. zuwa kasa da yuan 2,000 bayan an yi shawarwarin rage farashin, wanda zai inganta samar da maganin sosai.
A watan Agusta 2018, Patent Reexamination Board of State Intellectual Property Office ya yanke shawara na sake dubawa mai lamba 36902 buƙatun rashin aiki, kuma ta bayyana rashin ingancin ainihin ikon mallakar Pfizertofatib, haƙƙin mallaka, a kan dalilan rashin isassun bayyana ƙayyadaddun bayanai. Koyaya, sigar Pfizertofatiib crystal form (ZL02823587.8, CN1325498C, ranar aikace-aikacen 2002.11.25) zai ƙare a 2022.
Cibiyar Insight ta nuna cewa, baya ga binciken da aka yi na asali, an amince da wasu magunguna guda biyar na Chia Tai Tianqing, da Qilu, da Kelun, da Kogin Yangtze, da Nanjing Chia Tai Tianqing don yin tallace-tallace a cikin na'urorin kwamfutar hannu na tofacitinib na cikin gida. Koyaya, don nau'in kwamfutar hannu mai dorewa, kawai ainihin binciken Pfizer ya ƙaddamar da aikace-aikacen tallace-tallace a ranar 26 ga Mayu. Qilu shine kamfani na farko na cikin gida don ƙaddamar da aikace-aikacen talla don wannan tsari. Bugu da kari, CSPC Ouyi yana cikin matakin gwaji na BE.
Changzhou Pharmaceutical Factory (CPF) babban mai kera magunguna ne na APIs, wanda ya gama samar da magunguna a China, wanda ke Changzhou, lardin Jiangsu. An kafa CPF a cikin 1949. Mun sadaukar a Tofacitinib Citrate daga 2013, kuma mun ƙaddamar da DMF riga. Mun yi rajista a ƙasashe da yawa, kuma za mu iya tallafa muku tare da mafi kyawun tallafin takardu don Tofacitinib Citrate.
Lokacin aikawa: Yuli-23-2021